By Matthew Newton, DNP, CRNA
Medication administration in the clinical setting is one of the highest-risk responsibilities in healthcare, and errors in documentation, labeling, and storage can have profound consequences for patient safety, provider credibility, and institutional compliance. The World Health Organization estimates that medication errors contribute to over 42 billion dollars in healthcare costs annually and represent a leading cause of preventable harm worldwide. Within perioperative and anesthesia practice, the stakes are even higher due to the potency and immediacy of the medications being administered, combined with the numerous distractions of the operating room. Accurate and complete documentation is essential to safe practice. Charting errors, including incomplete entries or transcribing inaccuracies, account for up to 70% of medication-related mistakes. Clean, legible, and standardized records create a clear audit trail, ensuring accountability. At most facilities, waste must also be properly documented and cosigned, as missing or unexplained discrepancies are one of the leading triggers for pharmacy audits. These audits can have long-lasting implications for clinical practice. If discrepancies in drug use or volumes remain unaccounted for, pharmacies may adjust Pyxis dispensing limits or alter medication box configurations, changes that can directly disrupt workflow and patient care delivery.
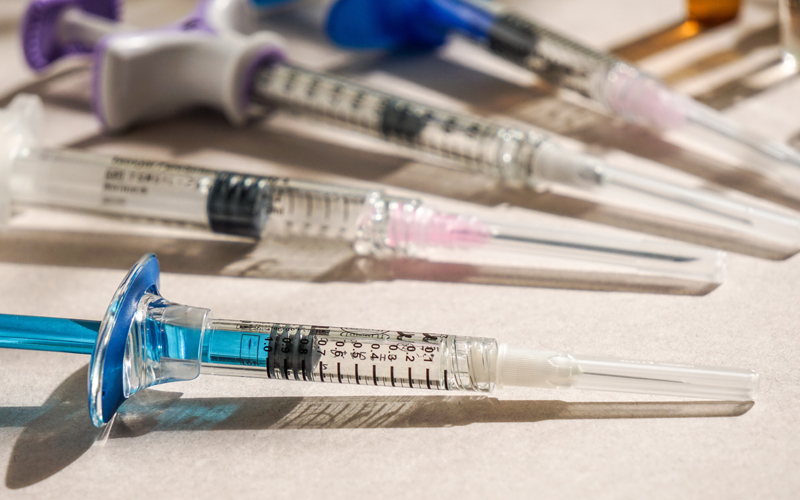
The fast-paced nature of perioperative care often places providers under pressure to expedite turnover. However, rushing significantly increases the risk of medication errors. One systematic review found that the risk of prescribing error increases by 30% when five or more drugs are administered concurrently. The clinical message is clear: taking an extra moment to double-check drugs, volumes, and documentation is not wasted time, but rather an investment in patient safety. Another critical safeguard is proper labeling. All prepared medications should be labeled with the drug name, concentration, date, time, and initials of the preparer. Proper labeling avoids misidentification, particularly when multiple syringes of similar appearance are present, and reduces the risk of administration errors during handoffs or team-based care.
Preparing medications in advance may appear efficient but it often introduces unnecessary risks. Syringes prepared outside of controlled environments demonstrate contamination rates as high as 16%. Additional risks include diversion, misplacement, and loss of sterility if carts are left unlocked inadvertently. For these reasons, medications should only be prepared on a case-by-case basis, immediately prior to use. Medication vials should also never be split unless explicitly designated as multi-dose, and syringes should never be refilled, even with the same medication for the same patient. The Centers for Disease Control and Prevention emphasizes that unsafe injection practices, such as reusing syringes or accessing vials with previously used syringes, can lead to life-threatening infections and cross-contamination. A simple “one drug, one syringe, one patient” rule remains the safest standard.
Finally, medication carts should always be locked when unattended Unsecured carts pose a risk of theft, tampering, or unauthorized use. Beyond compliance, this practice ensures that controlled substances remain fully accounted for, protecting both patient safety and provider integrity. Proper medication documentation and handling practices are non-negotiable elements of safe anesthesia care. From legible charting and signed waste records to careful labeling, single-use adherence, and cart security, each step reinforces patient safety and professional accountability. While the pressures of efficiency and turnover are ever-present, the extra minutes spent on deliberate, standardized practices prevent errors that can compromise patient outcomes and damage professional trust. By maintaining vigilance, providers not only reduce error risk but also strengthen the reputation of their teams and institutions, promoting a culture of patient safety.
References
• World Health Organization. (2017). Medication Without Harm: Global Patient Safety Challenge on Medication Safety. Retrieved from https://www.who.int/initiatives/medication-without-harm
• Mekonnen, A. B., et al. (2023). Documentation-related medication errors in clinical practice: A systematic review. BMJ Open Quality, 13(Suppl 1), e003188.
• Reynolds, P. M., MacLaren, R., Mueller, S. W., et al. (2009). Randomized trial of single- versus multi-dose vials for parenteral medications: Impact on contamination. American Journal of Health-System Pharmacy, 66(9), 865–869.
• Centers for Disease Control and Prevention (CDC). (2024). Injection Safety for Healthcare Professionals. Retrieved from https://www.cdc.gov/injection-safety